
HOW NINLARO® (IXAZOMIB)
WORKS

As the only oral proteasome inhibitor used to treat relapsed multiple myeloma NINLARO is different
NINLARO blocks the action of proteasomes, which break down unneeded proteins inside cells. By blocking proteasomes inside myeloma cells, NINLARO creates a buildup of proteins, causing the myeloma cells to die.
Proteasome inhibitors are a proven foundation for myeloma treatment. NINLARO is the first and only proteasome inhibitor that is taken orally. NINLARO is taken as part of an all-oral treatment combination*. NINLARO is not chemotherapy and does not require injections or infusions.
*NINLARO + lenalidomide + dexamethasone.
How NINLARO works inside myeloma cells
Learn how NINLARO specifically
targets proteasomes to help
destroy myeloma cells.
ViewHide Transcript
Multiple myeloma is a cancer of the bone marrow. Takeda has been engaged in the fight against multiple myeloma for nearly two decades.
The disease is characterized by the abnormal growth of cells called plasma cells [1] [2].
Inside the cell, the proteasome is something that helps get rid of a build-up of proteins [3].
Myeloma cells rely on the proteasome to survive [3].
Text appears on screen: Proteasome Inhibitors (PIs) stop cell growth and cause cell death.
By targeting the proteasome, these therapies may be able to stop cell growth, and cause cell death.
Text appears on screen: These therapies target the proteasome to treat multiple myeloma.
Treatments that target the proteasome are called proteasome inhibitors. They are a cornerstone of multiple myeloma treatment [3].
NINLARO® or ixazomib is a type of proteasome inhibitor [4]. NINLARO is a prescription medicine used to treat multiple myeloma in combination with the medicines REVLIMID® (lenalidomide) and dexamethasone, in people who have received at least one prior treatment for their multiple myeloma. NINLARO should not be used to treat the following people, unless they are participants in a controlled clinical trial: people who are receiving maintenance treatment, or people who have been newly diagnosed with multiple myeloma [4].
Text appears on screen: NINLARO® (ixazomib) may affect healthy cells.
As a proteasome inhibitor, NINLARO directly binds to the proteasome…
…helping to reduce its activity [4].
By stopping the proteasome, proteins begin to build up inside myeloma cells…
…and the build-up can cause death of the cancer cells [3].
Text appears on screen: NINLARO® (ixazomib) may affect healthy cells.
NINLARO is the first and only all-oral proteasome inhibitor [1].
NINLARO may be an alternative to other treatments for people whose multiple myeloma has come back for the first time [4].
If you have had at least one prior treatment for your multiple myeloma, your doctor may consider if the NINLARO regimen is right for you after your multiple myeloma first comes back [4].
Text appears on screen: Those that have had at least one prior treatment when multiple myeloma comes back for the first time.
The NINLARO regimen may be a good treatment option for you when your multiple myeloma comes back as a slow progressive disease with limited symptoms or you are a patient with an active lifestyle [1] [4].
Important Safety Information for NINLARO® (ixazomib)
NINLARO may cause serious side effects, including:
- Low platelet counts (thrombocytopenia) are common with NINLARO and can sometimes be serious. You may need platelet transfusions if your counts are too low. Tell your healthcare provider if you have any signs of low platelet counts, including bleeding and easy bruising.
- Stomach and intestinal (gastrointestinal) problems. Diarrhea, constipation, nausea, and vomiting are common with NINLARO and can sometimes be severe. Call your healthcare provider if you get any of these symptoms and they do not go away during treatment with NINLARO. Your healthcare provider may prescribe medicine to help treat your symptoms.
- Nerve problems are common with NINLARO and may also be severe. Tell your healthcare provider if you get any new or worsening symptoms including: tingling, numbness, pain, a burning feeling in your feet or hands, or weakness in your arms or legs.
- Swelling is common with NINLARO and can sometimes be severe. Tell your healthcare provider if you develop swelling in your arms, hands, legs, ankles, or feet, or if you gain weight from swelling.
- Skin reactions. Rashes are common with NINLARO. NINLARO can cause rashes and other skin reactions that can be serious and can lead to death. Tell your healthcare provider right away if you get a new or worsening rash, severe blistering or peeling of the skin, or mouth sores.
- Thrombotic microangiopathy (TMA). This is a condition involving blood clots and injury to small blood vessels that may cause harm to your kidneys, brain, and other organs, and may lead to death. Get medical help right away if you get any of the following signs or symptoms during treatment with NINLARO: fever, bruising, nose bleeds, tiredness, or decreased urination.
- Liver problems. Tell your healthcare provider if you get these signs of a liver problem: yellowing of your skin or the whites of your eyes; pain in your right upper-stomach area (abdomen).
Other common side effects of NINLARO include low white blood cell counts and bronchitis.
Tell your healthcare provider if you get new or worsening signs or symptoms of the following during treatment with NINLARO:
- skin rash and pain (shingles) due to reactivation of the chicken pox virus (herpes zoster)
- blurred vision or other changes in your vision, dry eye, and pink eye (conjunctivitis)
These are not all the possible side effects of NINLARO. Talk to your healthcare provider for medical advice about side effects. You may report side effects to Takeda at 1-844-217-6468 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Before taking NINLARO, tell your healthcare provider about all your medical conditions, including if you:
- have liver problems.
- have kidney problems or are on dialysis.
- are pregnant or plan to become pregnant. NINLARO can harm your unborn baby.
Females who are able to become pregnant:
- Avoid becoming pregnant during treatment with NINLARO.
- Your healthcare provider will do a pregnancy test before you start treatment with NINLARO.
- You should use effective non-hormonal birth control during treatment and for 90 days after your last dose of NINLARO. If using hormonal contraceptives (for example, birth control pills), you should also use an additional barrier method of contraception (for example, diaphragm or condom). Talk to your healthcare provider about birth control methods that may be right for you during this time.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with NINLARO.
Males with female partners who are able to become pregnant:
- You should use effective birth control during treatment and for 90 days after your last dose of NINLARO.
- Tell your healthcare provider right away if your partner becomes pregnant or thinks she may be pregnant while you are being treated with NINLARO.
- are breastfeeding or plan to breastfeed. It is not known if NINLARO passes into breast milk, if it affects an infant who is breastfed, or breast milk production. Do not breastfeed during treatment with NINLARO and for 90 days after your last dose of NINLARO.
Tell your healthcare provider about all the medicines you take, including prescription and over-the counter medicines, vitamins, and herbal supplements. Talk to your healthcare provider before starting any new medicines during treatment with NINLARO [4].
Taking too much NINLARO (overdose) can cause serious side effects, including death. If you take more NINLARO than instructed by your healthcare provider, call your healthcare provider right away or go to the nearest hospital emergency room right away. Take your medicine pack with you.
Please see Patient Information in the NINLARO full Prescribing Information.
NINLARO is the first and only oral medication of its kind. A proteasome inhibitor that goes where you go.
Have questions about NINLARO? Speak with your doctor to find out more.
REFERENCES:
- Raedler LA. Ninlaro (ixazomib): first oral protease inhibitor. Approved for the treatment of patients with relapsed or refractory multiple myeloma. Am Health Drug Benefits. 2016;9(Spec Feature):102-105.
- Mahindra A, Hideshima T, Anderson KC. Multiple myeloma: biology of the disease. Blood Rev. 2010;24 Suppl 1:S5-S11.
- Moreau P, Richardson PG, Cavo M, Orlowski RZ, San Miguel JF, Palumbo A, Harousseau JL; Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120(5):947-959.
- NINLARO. Prescribing Information. Takeda Pharmaceuticals America, Inc.; 07/2024.
Understanding NINLARO


Myeloma Cell
- Proteasomes act like a garbage disposal system, helping to clean up damaged or unneeded proteins inside cells
- In myeloma cells, there is a greater need for proteasomes to remove excess proteins that aren’t useful to the cell
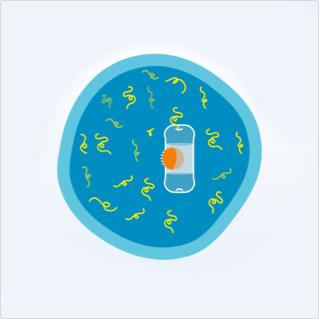
Proteasome inhibition with NINLARO
- NINLARO is a small molecule that enters the myeloma cell and binds proteasomes
- When it binds the proteasomes inside cells, it slows down or blocks proteasomes from doing their job of discarding damaged proteins within cells
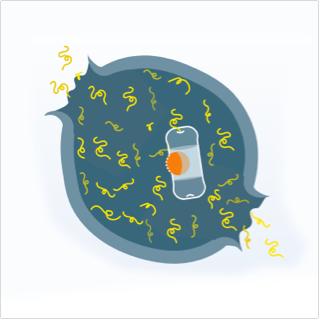
Proteasome inhibition effects
- Blocking proteasomes with NINLARO causes a buildup of proteins in the cell
- This leads to the death of myeloma cells
Results with NINLARO
Explore study data for NINLARO in combination
with lenalidomide + dexamethasone.
How to take NINLARO
Learn about taking the all oral
NINLARO treatment combination.